Corrosion in built assets

|
| The rust on this painted metal grille was probably caused originally by cracks in the paint coating leading to oxidation and rust of the metal which in turn caused the paint to flake off further and so continue the process. |
Corrosion is the gradual destruction of metals (mostly) due to natural processes, of which the main is oxidation, although there are others. It occurs when metals are exposed to the environment and are attacked by liquids or gasses whose actions instigate chemical reactions. The process can be either chemical or electrochemical. Ceramics and polymers are also subject to corrosion although the process is usually called ‘degradation’.
The rate and form of corrosion will depend on the type of metal in question (i.e how reactive it is) and the nature of the environment. The resulting chemical reaction converts the stable form of a metal to its oxide, hydroxide or sulphide, and thereby affects its strength, appearance and permeability to liquids.
The corrosion process usually involves a loss of electrons from the metal to form more stable compounds such as iron oxide – which has fewer free electrons to be taken away. Some metals are more inert and so have better natural resistance to corrosion, this is why metals such as gold and platinum are almost always found naturally in pure form. In contrast, iron, which is a lot less stable, is found mainly as iron ore (oxidised iron e.g haematite, magnetite, siderite) and requires refining (i.e reversing the corrosion process to remove the oxygen or other impurities) for use in a variety of applications.
One of the most common forms of the oxidation process is rust, which results from electro-chemical corrosion due to contact with moisture or polluted air, causing a metal, such as iron, to be converted into red-orange-coloured iron oxide and salts of iron, accompanied by a volumetric expansion.

|
| A green patina has formed on the copper cladding of this appartment building. |
With some metals such as steel, zinc and copper, the oxide that forms (sometimes called a ‘patina’) seals the surface and acts as a protective barrier against further corrosion. For example, copper roof sheeting acquires its characteristic green patina, while zinc sheet produces a grey-coloured patina. Steel oxidation results in an attractive ruddy-brown-coloured patina that has been prized by architects and is known as weathering steel. Used for a variety of architectural applications, particularly exposed steel structures, it is also self-healing when chipped or damaged. A well-known commercial variety is Cor-Ten, developed by the US Steel Corporation to obviate the need to paint steel but with the benefit of a tough surface.
Some metal alloys can corrode simply on exposure to air but the process can be exacerbated by the presence of warm temperatures and certain substances such as sulphates and other acidic agents. Some of these may be present in acid rain. However, alloys can have better inherent corrosion resistance than pure metals.
Corrosion occurs mainly on exposed surfaces which means that preventative measures such as paints and coatings can be applied to prevent or delay the onset of the process. Other protection methods such as galvanising and anodising can be applied in the factory before a product is installed.
[edit] Related articles on Designing Buildings
- Brittle fracture.
- Corrosion coupons.
- Corrosion inhibitor.
- Corrosion resistance.
- Corrosion resistant alloy CRA.
- Crevice corrosion.
- Deterioration.
- Dezincification.
- Galvanic corrosion.
- Graphitisation.
- Hydrogen embrittlement.
- Marine corrosion.
- Microbially Influenced Corrosion.
- Microbiologically influenced corrosion.
- Pepper pot corrosion
- Pitting.
- Rosette corrosion.
- Rust.
- Stress corrosion cracking.
- Under-deposit corrosion.
Featured articles and news
Apprenticeships and the responsibility we share
Perspectives from the CIOB President as National Apprentice Week comes to a close.
The first line of defence against rain, wind and snow.
Building Safety recap January, 2026
What we missed at the end of last year, and at the start of this...
National Apprenticeship Week 2026, 9-15 Feb
Shining a light on the positive impacts for businesses, their apprentices and the wider economy alike.
Applications and benefits of acoustic flooring
From commercial to retail.
From solid to sprung and ribbed to raised.
Strengthening industry collaboration in Hong Kong
Hong Kong Institute of Construction and The Chartered Institute of Building sign Memorandum of Understanding.
A detailed description from the experts at Cornish Lime.
IHBC planning for growth with corporate plan development
Grow with the Institute by volunteering and CP25 consultation.
Connecting ambition and action for designers and specifiers.
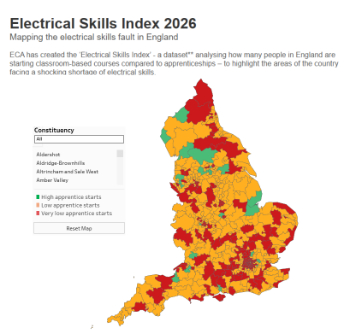
Electrical skills gap deepens as apprenticeship starts fall despite surging demand says ECA.
Built environment bodies deepen joint action on EDI
B.E.Inclusive initiative agree next phase of joint equity, diversity and inclusion (EDI) action plan.
Recognising culture as key to sustainable economic growth
Creative UK Provocation paper: Culture as Growth Infrastructure.
Futurebuild and UK Construction Week London Unite
Creating the UK’s Built Environment Super Event and over 25 other key partnerships.
Welsh and Scottish 2026 elections
Manifestos for the built environment for upcoming same May day elections.
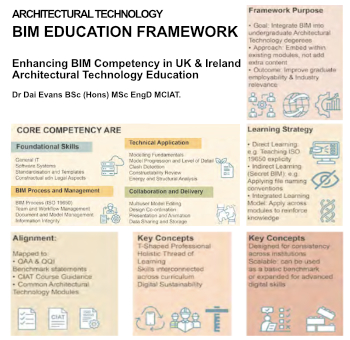
Advancing BIM education with a competency framework
“We don’t need people who can just draw in 3D. We need people who can think in data.”